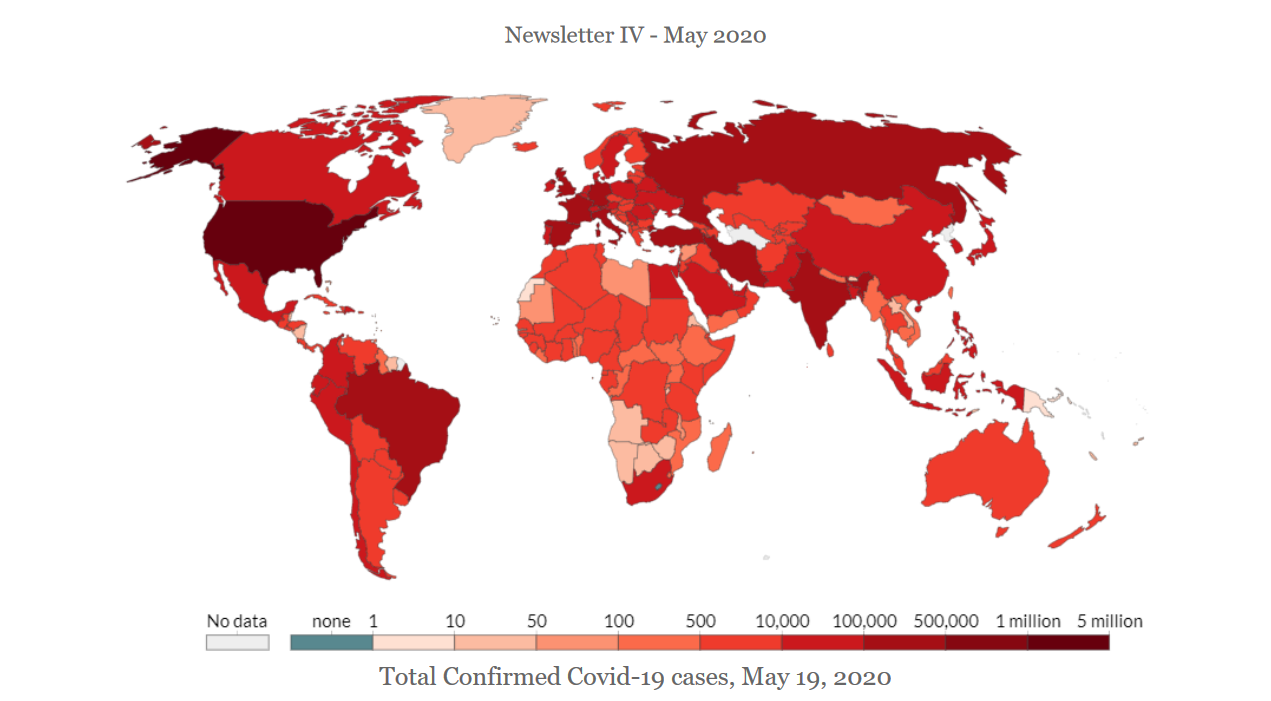
Ventilators are medical devices to help critical patients breathe in and breathe out. During COVID-19 pandemic, 1 out of 6 patients gets ventilators. However, owing to the rising numbers, hospitals are suffering from a dearth of ventilators even though the USA and European countries are producing them. Chinese ventilators were banned for being defective or for other reasons. In this newsletter, you will find FDA and EU CE mark regulations for ventilators. Under FDA, types of risks, types of devices and their classification, submission procedure, FDA process timeline, authorization process under EUA and duration authorization are discussed. Under EU Regulations, the medical devices are arranged as per their isk level, namely, high, medium, low and lowest. The newsletter also mentions temporary permits, an emergency application process for the UK.


